UHN Molecular Imaging Pipeline
Despite advances in screening, many cases of cancer go undetected until they have progressed to the point where treatment options are limited. The Molecular Imaging Pipeline at UHN aims to develop and translate new molecular imaging techniques for detecting and diagnosing cancer earlier and more accurately. By using specialized probes that interact with a patient’s biology, molecular imaging can further provide insight into disease processes and enable treatments to be precisely targeted and tailored to individual patients.
An approach that is gaining popularity in molecular imaging is the use of dual-purpose probes that enable imaging and the delivery of treatment. These probes are linked to radioisotopes, which emit therapeutic radiation and can be carefully targeted to cancer cells while sparing healthy tissues. This approach is part of the emerging field of theranostics, which combines therapeutics with diagnostics.
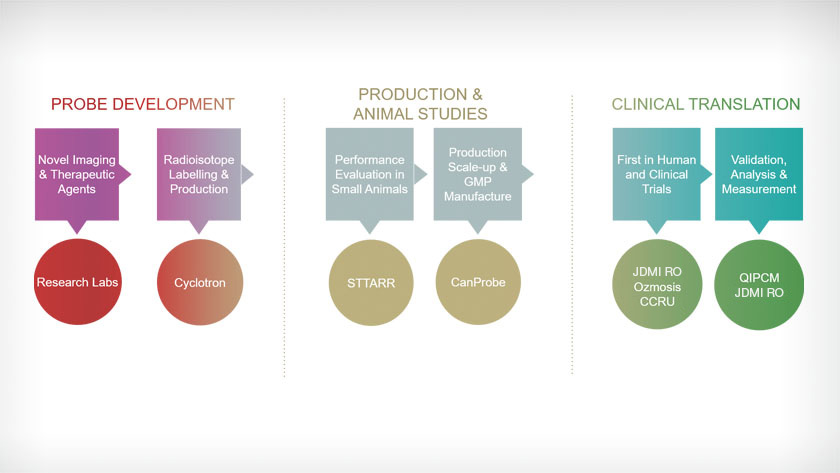
UHN’s Molecular Imaging Pipeline comprises three phases: Probe Development, Production and Animal Studies, and Clinical Translation. Several core facilities at UHN are integral to the pipeline.
To address cancers that are currently difficult to detect or treat, new diagnostic and therapeutic probes are needed. In the Probe Development phase research labs are developing probes to meet this need and are formulating processes for producing and attaching the functional radioisotopes. The new probes must then be tested in pre-clinical models of cancer and formulated for GMP production—the second phase. With PET, SPECT, CT, MR and other imaging technologies, the STTARR Innovation Centre provides an extensive research platform for the pre-clinical testing and evaluation of companion diagnostic and therapeutic probes. After pre-clinical testing, the probes are then scaled up for GMP level production at CanProbe , a cutting-edge cyclotron and radiopharmacy facility at UHN.
To translate the pre-clinical findings to clinical practice, first-in-human and other clinical trials are led that evaluate the safety and efficacy of the agents. This phase is facilitated by the Princess Margaret Cancer Clinical Research Unit (CCRU), and is carried out in coordination with the Joint Department of Medical Imaging (JDMI) Research Office, which provides imaging infrastructure such as PET-CT, PET-MR, and SPECT scanners for testing in patients. To coordinate multi-center trials, investigators work with the QIPCM Advanced Imaging Core Lab, which offers leading informatics infrastructure for centralizing, anonymizing, managing and analyzing images—all managed under strict privacy and quality standards which are imperative to regulatory compliance.
UHN is working pro-actively with industry and academic partners to assemble the infrastructure needed to grow this pipeline and make Toronto a hub for innovation in molecular imaging and theranostics. UHN already has tremendous expertise in radiopharmaceutical design, medical imaging, clinical translation, oncology, radiobiology, personalized dosimetry and image analysis— all of which are pre-requisites to a thriving molecular imaging and theranostics research environment and will be essential for supporting future growth.